Lead Investigator
Professor Alan Montgomery
Professor of Medical Statistics and Clincal Trials
University of Nottingham
Summary
Factorial trials, in which two or more interventions are simultaneously evaluated, have specific design and analysis considerations not covered by standard SPIRIT and CONSORT statements. Reviews show that the reporting quality of factorial trials is often poor.
The RAFT (Reporting Factorial Trials) study will develop SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) and CONSORT (Consolidated Standards of Reporting Trials) extensions for the reporting of factorial trials.
All rounds of the survey are now complete. We would like to thank everyone for their support.
Factorial trials
Factorial trials, in their simplest form (2x2), are when two interventions (A and B) are tested. For example, participants are randomised to receive: no intervention, A alone, B alone or both A and B.
Factorial trials offer an efficient method to evaluate multiple interventions without increasing the sample size, provided the treatments work independently. The focus of the RAFT study is on factorial designs which aim to efficiently assess multiple interventions in a single trial.
Some keyword definitions that are frequently used in factorial trials can be found
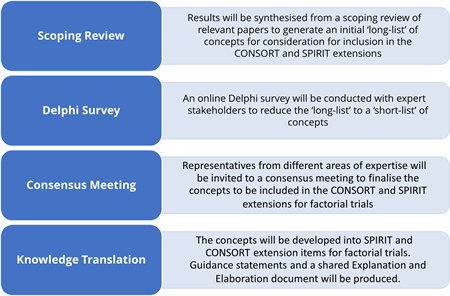
Components of the RAFT study
If you have any enquires, please get in touch with us via email:
Study updates
- All 3 rounds of the survey are now complete
- The RAFT consensus workshop took place in Nottingham on the 6th and 7th September
- Current publications:
CONSORT: https://jamanetwork.com/journals/jama/fullarticle/2812475
SPIRIT: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2812568
Invited commentary: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2812567
Useful links